Abstract
Introduction:
The second-generation proteasome inhibitor carfilzomib (K) was initially approved in the United States (US) in 2012 as a single agent for treatment of patients with MM who received ≥2 prior therapies, including bortezomib and an immunomodulatory agent. K was subsequently approved in combination with lenalidomide and dexamethasone (KRd; in 2015) or with dexamethasone (Kd; in 2016) for treatment of patients with relapsed or refractory MM who received 1-3 prior lines of therapy. Due to the many regimens and dosing options available, we evaluated patient characteristics according to regimen and line of therapy (LOT). This study describes the demographic and disease characteristics of patients receiving K-based regimens in clinical practice since its approval.
Methods:
Data analyzed were from a database of oncology electronic health records (EHR) contained in Oncology Services Comprehensive Electronic Records (OSCER) database, generated by Flatiron Health (New York, NY, April 30, 2016). OSCER represents a longitudinal, demographically and geographically diverse database containing data from over 265 cancer clinics representing over 1.7 million active patients treated at community-based hematology/oncology practices and from three academic centers in the US. Patients identified were aged ≥18 years, diagnosed with MM (ICD9: 203.x; ICD-10: C90.x) and initiated K-based therapy in the years 2012-2016. Patient demographic and disease characteristics were identified, as well as the year of therapy initiation, LOT (1-4), and all treatment regimens received. Five treatment regimens were identified: four regimens based on K dosed at 27 mg/m2 twice weekly (Kd27, Kd27 + lenalidomide [KRd], Kd27 + pomalidomide [KPd], and Kd27 + cyclophosphamide [KCyd]) and Kd with K dosed at 56 mg/m2 (Kd56) twice weekly, as well as any other treatment combination with K. All regimens were assumed to contain dexamethasone.
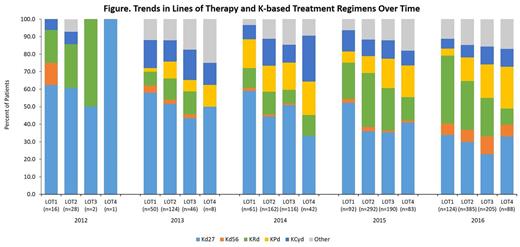
Results:
Out of a total of 30,143 MM patients, 2202 K-treated patients were identified. Among K-treated patients, 40.6%, 36.7%, and 22.6% were aged ≤65 years, >65 and ≤75 years, and >75 years of age at index data, respectively, with 58.3% male and 65.7% white. Detailed results of the patients' clinical characteristics will also be presented. Kd27, the first approved K-containing regimen, was the most common K-based treatment regimen across all years, but the proportion of use relative to other K-based regimens was highest in 2012-2014 (61.7%, 51.3%, and 47.7% of patients, respectively), and decreased over time as other K regimens became available (39.1% and 29.5% in 2015 and 2016, respectively). KRd was the second-most common K regimen, representing 24.4% and 25.3% of K regimens in 2015 (year of approval) and 2016, respectively. Kd27 and KRd were also the most common treatment regimens across LOT (see figure). Use of Kd56 grew over time; in 2016 it accounted for 6.5%, 7.0%, 10.2%, 6.8% of LOT1-4, respectively. Additionally, over time, triplet regimen (KRd, KPd, KCyd) use increased. In 2012-2014, these triplet regimens were 29.8%, 32.5%, and 40.1% of all K regimens, respectively. Triplet use increased to 46.9% and 47.1% by 2015-2016.
Conclusion:
In 2012-2014, real-world use of K was primarily at the 27-mg dose in combination with dexamethasone. In 2015-2016, treatment patterns shifted, with KRd and Kd56 being used in a higher proportion of K-treated patients. This pattern may continue to grow since data from the phase III trials of these regimens showed improved survival. Drug combinations of K with pomalidomide or cyclophosphamide were used during the entire study period. Use of K-based triplet regimens increased over time. Overall, use of K-based regimens continues to grow, and the regimens in which K is used are changing with emerging data and as the understanding of the disease management evolves over time.
Kim: Amgen, Inc.: Employment, Equity Ownership. Werther: Amgen, Inc.: Employment, Equity Ownership. Iskander: Amgen: Employment, Equity Ownership. Gruber: Amgen, Inc.: Employment, Equity Ownership. Mezzi: Amgen, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal